pp. 35-41
Hariom Nagar
Suresh Gyan Vihar University, Jaipur-302017, Rajasthan, India.
Email: hariomnagariitr@gmail.com, hariom.nagar@mygyanvihar
ABSTRACT: Amino alcohols like β-blockers are very important drugs for the treatment of heart related diseases. This is due to presence of active functional groups (-OH and NH2) in their unique and simple structure. Betaxolol a selective β1-receptor antagonist is one of the most prescribed drug for treatment of particularly hypertension, and it is also a very strong antiglaucoma agent. (S)-(−)-enantiomer of β-blockers shows about 50-500 fold higher pharmacological activities, due to the specific and proper orientation of groups to react with β1- receptor. There has been no attempt to review on the enantiomeric separation of betaxolol using chromatographic techniques. This paper deals with the application of: high-performance liquid chromatography by direct method, using chiral stationary phases and, by indirect method using chiral derivatizing reagent and; thin layer chromatography by direct method using chiral selector as chiral impregnating reagent, chiral inducing reagent and chiral mobile phase additives.
Keywords: β-blockers, chiral selector, enantiomeric separation, thin layer chromatography, high-performance liquid chromatography, chiral derivatizing reagent
INTRODUCTION
β-blockers (also known as β-adrenolytic drugs) consist of a group of synthetic chiral hydroxyl amine-containing compounds with pKa ∼ 9.2. They stop adrenaline from binding to nerve receptors and “block” its effects. Betaxolol a potent β1- receptor blocker used in the treatment of hypertension and glaucoma. It shows greater affinity for β1-receptor than metoprolol. The (S)-( ̶ )-enantiomer of betaxolol drug (Figure) is pharmacologically effective, showing about 50–500-fold higher activities (Lee and William 1990). In most cases betaxolol is administered as racemic mixtures. Since the enantiomers have different activity in ‘chiral environment of our body’ therefore these enantiomers are considered different compounds. Many biologists and clinical pharmacologists tend to deal with enantiomeric mixtures of isomers. A physician, presenting such a drug under a brand name is unaware of different activity of enantiomers and may make mistakes (Ariens 1984). Therefore, many regulatory agencies ask the pharmacologists to present full pharmacokinetics and pharmacological information of both the enantiomers. Because in certain cases, the less reactive or unwanted enantiomer can have side effects or even toxic effects. Therefore, efficient methods of enantioresolution are always required to control the enantiomeric purity or to resolve racemic mixtures and to determine optical purity of the drugs.
Enantiomeric separation has immense importance in various fields from academic and industrial point of view. For enantiomeric separation two basic strategies ‘‘direct approach’’ and ‘‘indirect approach’’ have evolved in recent years. The direct approach has no require derivatization and separation can be achieved by using chiral selector: as chiral impregnating reagent, chiral mobile phase additives and chiral inducing reagent in thin layer chromatography and; as chiral stationary phase in high-performance liquid chromatography, to produce chiral environment in situ formation of transient diastereomers between enantiomers present in analyte and the enantiomer of pure chiral selector. In indirect method, there is first conversion of the enantiomers of analyte species in to daistereomers by reacting them with chiral derivatizing reagents and then separation can be done by chromatography in achiral environment.
In literature review there have been reported some basic approaches and principles of planar and column chromatography for enantiomeric separation of betaxolol. Nevertheless, there has been no attempt to review on the enantiomeric separation of betaxolol by chromatography using the direct and indirect approaches. The present paper focused on the use of liquid chromatography for the enantiomeric separation of betaxolol.
DIRECT APPROACH
Ennatiomeric separation using direct approach is depends on the chiral environment which is either produced by incorporating a suitable chiral selector at an appropriate stage or created in the form of chiral stationary phase (CSP). This chiral invironment can be created in thin-layer chromatography (TLC) and high- performance liquid chromatography (HPLC) by (a) preparing the TLC plate or the column with the chiral selector, (b) impregnating the chiral selector on stationary phase of TLC, or (c) adding the chiral selector to the mobile phase. The material mentioned at (a) is chiral owing to its own structure (e.g. cellulose) while the material mentioned at (b) is prepared by bonding the chiral selector of interest to reactive groups of inert support. In situation (c) chiral environment is created by mixing the chiral selector and with mobile phase and flow of this mixture prior to separation. In all the cases, transient daistereomers are formed on the surface by the interaction of chiral selector with enantiomers of analyte of interest. The separation of these enantiomers is based on the concept is that, the enantiomer forming most stable daistereomers will be most retained with stationary phase it means elute later and the enantiomer forming less stable daistereomer elute earlier. Martens and Bhushan (1990) direct methods for separation of enentiomers are important in various fields like synthetic and mechanistic study, pharmaceutuical and biomedical analysis.
Enantiomeric separation by TLC
Using Impregnation
The technique in which a suitable chiral selector is incorporating with the adsorbent without covalently affecting its inert character, owing to application of samples on the plate for their separation is termed as impregnated TLC. There are various methods used for impregnation of TLC: spraying the solution of the impregnating reagent on the plate; immersing plain plate or ascending or descending the plate in a normal manner of development into an appropriate solution of the impregnating reagent; exposing the thin layer to the vapors of the impregnating reagent; and mixing the chiral selector with the inert support. In case of immersion there may be peeling of the thin layer, and in spraying method less uniform dispersion of the chiral selector on inert support than in the mixing of chiral selector with inert support or immersion (Bhushan and Martens, 1997).
The overall results of resolution are depends on the kind of the functional groups and chemical nature of the impregnating reagent the compounds being separated, along with the influence of the impregnating reagent on the partition/or adsorption. The methods used for impregnation also play important role in the resolution of the enantiomers.
This method provides an inexpensive and wide choice of chromatographic conditions for enantiomeric separation of variety of compounds. Enantiomeric separation of betaxolol has been carried out using the L-lysine/L-arginine (Bhushan and Thiong’O, 1998), as chiral impregnating reagent and chromatographic data in terms of resolution and limit of detection (LOD) are given in Table.
Using chiral inducing reagent (CIR)
CIR: The chiral selector when mixed with the racemic mixture of the analyte then itself induced the chiral environment in medium prior to formation of transient daistereomers of analyte, so it is called the chiral inducing reagent. The advantages of this method over the impregnation is that the required quantity of the chiral selector is very less than the quantity of the chiral selctor required in impregnation methods. Since, in this method no requirement to produce chiral environment on the inert support of TLC for separation of enantiomers. Therefore, the TLC is called as achiral TLC and the separation is known as achiral phase chromatographic separation.
Literature reported the use of (S)- Glutamic acid as CIR and chromatographic run was performed, results obtained were good in terms of RS (Bhushan et al. 2015).
Enantiomeric separation by HPLC
It is most widely used method for the enantiomeric separation. Several chiral columns have been used in recent years for enantiomeric separation. These chiral column are commercially available in market. This method gives better result than the TLC method in regard of accuracy, precision and detection limit of the analyte. The detectors generally used in this technique is photodiode array detector (PDA) 2600, make the detection of analyte molecules up to pico meter level. Several columns containing chiral stationary phases, based on: cellulose, amylose, pirkle, used for enantioseparation. Of the above stationary phase based columns, certain have been used for enantioseparation of betaxolol; Cellulose tris(3,5-dichlorophenyl-carbamate) (Ates et al. 2013), Cellulose tris(3,5-dimethylphenyl- carbamate), (Peng et al, 2010), Amylose tris(3,5-dimethoxyphenyl carbamate), (Cass et al. 1999), and comparative data in terms of resolution time, limit of detection and resolution values, are given in Table.
INDIRECT APPROACH
Chromatographic conditions of indirect method can be optimized more easily than the direct method, therefore, separation of daistereomeric pair via indirect method is sometimes simpler and often better resolution obtained. High sensitive detector response in this technique is providing better determination of biological samples in blood and urine. The CDR used for derivatization of samples are generally chosen with good limit of detection. For example the Marfey’s based CDRs shows high sensitive detector response to samples because of high molar UV-Vis absorptivity (ɛ) and fluorescence quantum yield. The reaction of CDR react with enantiomers of (±)- or the (R,S)-mixture is of different rates, therefore derivatization conditions must be optimized. In the structure of analyte sample suitable reactive group should be in close proximity to the stereogenic center, which is prone a quantitative transformation with the CDR and without forming the side products. Racemization must not be there of the sample and of the CDR in the reaction, which transforms the enantiomeric mixture into daistereomers.
Enantiomers of betaxolol has been separated by converting them into daistereomers. Several CDRs have been used for the formation of daistereomers of betaxolol prior to their separation by HPLC. In Table separation data are given in terms of ‘‘resolution’’ of daistereomers of betaxolol formed with following CDRs: (–)- (α-methoxy-(α- (trifluoromethyl)phenylacetyl chloride (Kim et al. 2001); 1,3-diacetoxy-1-(4-nitro- phenyl)-2-propyl isothiocyanate (Péter and Fülöp, 2001); 2,3,4,6-tetra-O-acetyl-b-D- galactopyranosyl isothiocyanate (Ko et al. 2006); 2,3,4,6-tetra-O-acetyl-b-D- glucopyranosyl isothiocyanate (Ko et al. 2006). Marfey’s reagent based CDRs; (R)- 2-(5-fluoro-2,4-dinitrophenylamino)-3- (methylthio)propanoic acid, (S)-N-(1- cyclohexylethyl)-5-fluoro-2,4- dinitrobenzenamine, (R)-5-fluoro-2,4- dinitro-n-(1-phenylethyl)benzenamine, have been used by Bhushan and Nagar (2014) for synthesis of daistereomers of betaxolol and the structures of these daistereomers also optimized by using 09 Rev A. 02 and hybrid density functional B3LYP with 6-31G basis set.
CONCLUSIONS
The liquid chromatography using direct and indirect methods are very useful for quantitative and qualitative analysis of important pharmaceuticals. Direct method is more suitable for separation of enantiomers in their pure form without any derivatization and is less time consuming. Direct TLC method (i) is less expensive in comparison to HPLC, GC and capillary electrophoresis that require highly expensive experimental set-up and running costs, (ii) is successful for rapid and sensitive resolution, detection and isolation of enantiomers in a range lower than the limits prescribed (1%) for pharmaceuticals in industry. Direct HPLC is widely used method for separation of enantiomers because (i) most of the CSPs are commercially available in market, (ii) detection and isolation limit is up to pico meter level, (iii) no requirement of derivatization hence less time consuming. But the chiral column is of high cost in direct method, while in indirect method no requirement of chiral column so indirect HPLC is less expensive and therefore more useful for detection of enantiomers.
ACKNOWLEDGEMENT
The author (H Nagar) is grateful to SGVU, Jaipur for providing the technical facilities and peaceful environment for doing this job.
REFERENCES
Lee, E.J. and William, K.M. (1990). Chirality: Clinical pharmacokinetic and pharmacodynamic considerations. Clin. Pharmacokinet. 18: 339–345.
Ariëns, E.J. (1984). Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur. J. Clin. Pharmacol. 26: 663–668.
Bhushan, R. and Thiong’O, G.T. (1998). Direct enantioseparation of some β- adrenergic blocking agents using impregnated thin-layer chromatography. J. Chromatogr. B 708: 330-334.
Bhushan, R. and Martens, J. (1997). Direct resolution of enantiomers of impregnated TLC. Biomed. Chromatogr. 11: 280-285.
Bhushan, R., Nagar, H. and Martens, J. (2015). Resolution of enantiomers with both achiral phases in chromatography: conceptual challenge. RSC Adv. 5: 28316- 28323.
Ates, H., Younes, A.A., Mangelings, D., and Heyden, Y.V. (2013). Enantioselectivity of polysaccharide- based chiral selectors in polar organic solvents chromatography: Implementation of chlorinated selectors in a separation strategy. J. Chromatogr. Biomed. 74: 1–13.
Peng, L., Jayapalan, S., Chankvetadze, B., and Farkas, T. (2010). Reversed- phase chiral HPLC and LC-MS analysis with tris(chloromethylphenylcarbamate) derivatives of cellulose and amylose as chiral stationary phases. J. Chromatogr. A 1217: 6942–6955.
Cass, Q.B., Tiritan, M.E., Calafatti, S.A., Matlin, S.A. (1999). Enantioseparation on amylose tris (3, 5-dimethoxyphenyl carbamate): application to commercial pharmaceutical chiral drugs. J. Liq. Chromatogr. Rel. Technol. 22 3091–3099.
Kim, K.H., Lee, J.H., Ko, M.Y., Hong, S.P. and Youm, J.R. (2001). Chiral separation of β-blockers after derivatization with (–)-a-Methoxy-a- (trifluoromethyl)phenylacetyl chloride by gas chromatography.
Arch. Pharm. Res. 24: 402–406. péter, M. and Fülöp, F. (2002). Comparison of isothiocyanate chiral derivatizing reagents for highnperformance liquid chromatography. Chromatographia 56: 631–636.
Ko, M.Y., Shin, D.H., Oh, J.W., Asegahegn, W.S. and Kim, K.H. (2006). Chiral separation of βnblockers after derivatization with a new chiral derivatization agent, GATC. Arch. Pharm. Res. 29: 1061–1065.
Bhushan, R. and Nagar, H., Enantioseparation of orciprenaline, betaxolol, and propranolol using HPLC and new chiral reagents based on 1,5-difluoro-2,4-dinitrobenzene. Anal. Lett. 29 (2014) 202-219.
Figure
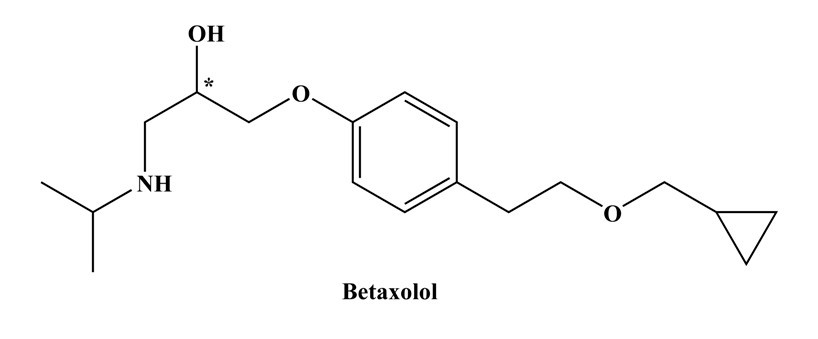
Table Literature reports with present study on chromatographic separation (in terms of: RS, LOD and resolution time) of enantiomers of Bel using different CSPs/chiral selectors/CDRs/CIR
| S. CSPa/CDRc/Chiral Selectorb/CIRd Technique Resolution time LOD RS Reference
No. used (tR/k/run time) |
| 5 Cellulose tris(3,5-dichlorophenyl- i NA NA 0.81 23
carbamate) a |
| 6 Cellulose tris(3,5-dimethylphenyl- i 15.2/16.9 min (tR1/tR2) NA 1.6 24
carbamate) a |
| 7 Amylose tris(3,5-dimethoxyphenyl i 11.8/14.8 min (tR1/tR2) 10 µg/mL 1.54 25
8 carbamate) a 31.7/32.9 (tR1/tR2) 26 |
| (–)-(α-methoxy-(α-(trifluoro- ii 19.8/20.0 NA 1.18 Kim 2001)
9 methyl)phenylacetyl chlorideb (k1/k2) |
| 1,3-diacetoxy-1-(4-nitro-phenyl)-2-propyl 4.67/5.58 (k1/k2) 0.2nmol/ 1.62
10 isothiocyanateb mL Péter, ii 2001 |
| 2,3,4,6-tetra-O-acetyl-b-D-galactopyranosyl ii NA NA 6.32 (Ko, et al, isothiocyanateb 2006) |
| 2,3,4,6-tetra-O-acetyl-b-D-glucopyranosyl ii NA NA 4.70 (Ko, et al,
13 isothiocyanateb 2006) |
| 14 (R)-2-(5-fluoro-2,4-dinitrophenylamino)-3- ii 21.48/23.88 (tR1/tR2) 12pgmL-1 11.72 (Bhushan
(methylthio)propanoic acidb and and Nagar 13pgmL-1 2014) |
| (S)-N-(1-cyclohexylethyl)-5-fluoro-2,4- ii 34.71/36.92 (tR1/tR2) 12pgmL-1 9.65 (Bhushan
dinitrobenzenamineb and and Nagar 13pgmL-1 2014) |
| (R)-5-fluoro-2,4-dinitro-n-(1- ii 33.64/35.63 (tR1/tR2) 12pgmL-1 8.70 (Bhushan
phenylethyl)benzenamineb and and Nagar 13pgmL-1 2014) |
| L-lysine/L-argininec 5.50/6.45 (k1/k2) NA 2.0 Bhushan
14 and Thiong’O, iii 1998 |
| 18 iii 1.2-1.8 µg 2.0 (Bhushan L-Glutamic acid d 10 min (run time) et al.
2015) |
| CSP, chiral stationary phase; CDR, chiral derivatizing reagent; CIR, chiral inducing reagent; tR1 and tR2 are the retention time of first and second eluting isomer, respectively; k1 and k2, retention factor of first eluting isomer; run time, time taken by solvent system to migrate across the TLC plate. i, ii and iii represent the techniques used as direct HPLC, indirect HPLC and direct TLC separation, respectively. |
