Research Paper
SGVU Journal of Pharmaceutical Research & Education
Journal homepage: http://www.gyanvihar.org/researchjournals/
Formulation, Evaluation & Optimization Study of Mucoadhesive Microspheres of Flurbiprofen Prepared By Spray Dry Method
Prashant B Patil * 1, Santosh K Singh 1, Girish A. Kashid2
Department of Pharmaceutics 1 , Department of Pharmaceutical Chemistry2 School of Pharmacy Suresh Gyan Vihar University Jaipur – 302017, Rajasthan, India
ABSTRACT:
The utility of microspheres to deliver drugs shows various advantages, such as control release of drugs, enhances bioavailability & site-specific administration of the drug to the required location. This study work showed the use of encapsulation of sodium alginate, and sodium carboxy methyl cellulose in a microparticulate drug delivery system, which deliver orally by a capsule & gives the required therapeutic action. A microsphere preparation shows merits on the conventional tablet & capsule formulations, this formulation improves surface area to enhance absorption of the drug in the specific area & also reduces the frequency of drug dose. Flurbiprofen (NSAIDs) drug preferably use in different intestinal diseases colon ulcers, different colon cancers & infections. Flurbiprofen proved more absorption from lower GIT regions, & also showed t1/2 4 hrs., orally it gives less bioavailability. The microsphere preparations were characterized & evaluated for yields of production, drug content (actual), efficiency of encapsulation, percentage Swelling Index, drug release study was done by in vitro release examination, mucoadhesive strength determination in vitro & in vivo methods.
Keywords: Mucoadhesive, Microsphere, Flurbiprofen.
INTRODUCTION
The idea of the mucoadhesive system came from the requirement to localize drugs to a specific site in the body for a prolonged period of time. Need because of the residence time of the drug in the absorption site.
In oral drug delivery, the absorption of the drug in the absorption site is less due to the GIT transit time of the dosage form. To illustrate suppose if a drug dosage form is to deliver a drug in a sustained manner for treating some chronic disease then it is required that the dosage form should remain at the site of drug absorption which is mainly due to the upper part of the intestine, for an extended period of time but this is limited because of the GI transit of the dosage form, so mucoadhesive dosage forms are formulated with the purpose of binding with the GIT mucus layer & thus improves the staying time of the drug and also providing the long time contact between a dosage form & absorbing tissue and hence enhancing the absorption of the drug1-3.
MATERIALS AND METHODS:
Flurbiprofen provided by Teva Pharma Pvt. Ltd., also Sodium alginate & Sodium carboxy methyl cellulose gift sample from Colorcon Ltd., UK. The optimization study was done by use of Design-Expert software, version 7.0.0. MS Excel, PCP disso. Pune software was used for the study of drug release analysis. Also prepared microspheres were characterized & evaluated for Yield of production, drug content (actual), efficiency of encapsulation, percentage Swelling Index, and drug release study.
Preparation of Microspheres
Spray dray technique:
Spray drying techniques were used to formulate mucoadhesive microspheres. An aqueous Phase incorporating various combinations of polymers (Table 1) was formulated by dissolving sodium alginate and carboxymethylcellulose in the distilled deionized water. The drug qty (1 g), in previously dissolved 100 ml of absolute methanol & was added to polymer solution & sonicated by using a sonicator (Ultra 1204 AU-Vibracell USA) to obtain a uniform mixture. Glutaraldehyde (0 – 0.30 ml) was used as a crosslinking agent, was added to the homogenized solution & produce solution was spray-dried by using (LU-222 ADVANCED) lab spray drier (Labultima, In) for Formulating microspheres through the nozzle of a spray-dryer ( model JISL, LSD- 48 mini spray dryer, In) with at input temperature range of 115 -117 °C, & output temperature range of 80 – 85 °C at 2 % feed rate & vacuum pressure of 35 psi (2.4 kg/cm2). The prepared microsphere was collected from a spray dryer & place in a desiccator including silica gel for the remaining further tests4-6.
Factorial formulations
Table.1 Combination batches by using Sodium alginate & CMC in various concentrations according to 32 factorial designs.
| CONTENT weight (mg) | F.1 | F.2 | F.3 | F.4 | F.5 | F.6 | F.7 | F.8 | F.9 |
| Flurbiprofen: Sodium alginate: Sodium CMC | 1:2:0 | 1:2:1 | 1:2:2 | 1:2:0 | 1:2:1 | 1:2:2 | 1:2:0 | 1:2:1 | 1:2:2 |
| Cross-linking agent (%) | 00 | 00 | 00 | 20 | 20 | 20 | 30 | 30 | 30 |
Factorial Batches 7-10: –
A factorial design 32 was implanted for the optimization of oral controlled release mucoadhesive microspheres. According to this model, it contains 02 independent variables at three levels +1,0 and -1. total nine formulations possible with this model. The content of different formulations is shown in (Table.2). The various independent variables include drug: polymer ratio (X1) & also % of Cross linking agent (X2), where carboxyl methyl cellulose & sodium alginate act as controlled-release polymers. The different dependent responses include % drug release at 8 hours (Y1), Time taken to release 50% drug, T50% (Y2), and Time taken to release 90% drug, (Y3 ).
Combination Batches for microspheres: –
Table.02 Factorial Design (Preparation of Microspheres Batches)
| Batch Code | Variable levels with Coded form | |
| X1 | X2 | |
| F.1 | + 1 | + 1 |
| F.2 | + 1 | 0 |
| F.3 | + 1 | – 1 |
| F.4 | 0 | + 1 |
| F.5 | 0 | 0 |
| F.6 | 0 | – 1 |
| F.7 | – 1 | + 1 |
| F.8 | – 1 | 0 |
| F.9 | – 1 | – 1 |
X1 : drug: polymer (ratio) X2 : Cross-linking agent(Concentration)
EVALUATIONS OF MICROSPHERES:
- Yields of production11-14
Production yields of microspheres for various batches were determined by using after drying mass of the final product with respect to the initial total weight of the product & polymer were used for the preparation of microspheres & % production yields were determined by the formula given below & results section results are reported.
Yield of Production (%) = Practical weight(microspheres) X 100……………..1
Theoretical weight (polymer & drug)
- Actual drug content and encapsulation efficiency11-14
The cacl2 solution in which the microspheres were prepared were calculated for its actual drug content by UV spectroscopy by taking its absorbance at 247nm & amount of unentrapped drug was estimated, then after the determined amount of drug was deducted from the total quantity of initial drug added to obtain the amount of drug which is entrapped(encapsulated). Encapsulation efficiency was estimated by using a direct method in which the microspheres were added in water for 24 hrs with constant shaking with this we can extract the drug from microspheres in water, which is then quantitatively determined by UV spectroscopy by taking its absorbance at 247nm & obtained used to determine encapsulation efficiency for the microspheres & using formula mentioned below & encapsulation efficiency values were shown in the results section.
% encapsulation efficiency = Actual drug content(mg) X 100……..2
Total wt. of microspheres
- Morphology of microspheres11-14
The microsphere’s size & shape for the optimized batches were determined through an optical microscope and through SEM (camera, France model-SV30). Results are reported results.
- Swelling studies11-14
The swelling index of the mucoadhesive microspheres in the physiological media was determined by adding 500mg of microspheres estimated by adding in pH 6.8 phosphate buffer (100ml) of & kept for 24hrs & equations were used to determine the ability of swelling.
S.s.w = (W.s – Wo/Wo) x 100 …………………… 3
Where S.s.w = % swelling of microspheres,
W.o = initial wt. of microspheres, W.s = weight
of microsphere after swelling.
- In vitro release study15-19:
In vitro release was studied for the drug by dissolution method using dissolution apparatus I (basket). The release study was performed by using 900 mL (v) pH 1.2acidic buffer. The temperature was constant at 37 ± 0.5°C & speed of the basket was at 100 rpm in the dissolution release study. Microspheres filled in capsule and placed in dissolution medium. At appropriate sampling time intervals, withdrawal of 5 mL of the solution & filtered taken absorbance of all samples was determined on a UV spectrophotometer (Jasco V-630, Japan) at 224 nm, with maintaining sink condition in the apparatus. In triplicate performed this study. The % drug release was measured by PCP disso software & reported in the results.
Study of release mechanism by Curve fitting:-
Release data were put into various mathematical models to determine which release mechanism from mucoadhesive microspheres; Korsmeyer Peppas (Eq. (4)), zero-order (Eq. (5)), and Higuchi release models (Eq. (6)). And reported in the results.
Mt/M∞ = kKPtn …………………………………………… 4
Where M t/M ∞ – fraction (drug released at time‘t’)
kKP – constant(release rate)
n – release exponent.
Mt = M0 + k0t……………………………………………… 5
Where Mt – Amount (drug released at time‘t’)
M0 – concentration (drug in the solution at t=0)
k0 – release constant (zero-order).
Mt = kH t1/2 ……………………………………………………. 6
Where, Mt – Amount (drug release at time ‘√t’)
kH – Higuchi release constant.
All curve fitting, simulation, and plotting were carried out by using disso software (PCP V3). The mechanism of the drug release is discussed in the results.
- In vitro mucoadhesion strength determination of microparticles20-22:
A recently excised sheep’s stomach was used. Before the study tissue mucus surface was washed with the saline normal water & tissue inclined at a 60o angle using polyethane support. A glass beaker was inserted directly under polyethane for microparticles collection when they detached from tissue. A 100 mg weight of microparticles prepared in different combinations of polymers were inserted on the trough of the mucus surface & permit to hydrate for 15 minutes for interaction between the microparticle–mucin to occur. A 100 ml vol of SGF was permitted to flow over tissue at a rate of 40 drops/minutes. The weight of microparticle washed out determined as a % of the original weight was used as a measurement of mucoadhesion. And results are reported.
- In vivo studies23-25:
- Weight count method
In this technique, 5 groups of 4 Albino rats overnight fasted & 100mg suspension of microspheres administer via needle to these rats, then after these rats sacrifices with an interval of 0, 4, 8, 12 hrs respectively. Then dissect their stomach area isolate & cut open longitudinally & note the weight of microspheres adhering to the stomach and intestine area, adhesive strength is determined by using the formula given below.
% adhesive strength = N.o – N.s X 100 ……………………….7
N.s
Were, No = Weight of microspheres hydrated with a small amount of H2O
Ns = Weight of microspheres detaching from the mucosal surface.
And results are reported.
RESULTS:
Table.3 Factorial batches dissolution studies of spray dry method
| Formulations | ||||||
| F1 | F2 | F3 | F4 | F5 | ||
| * Percent drug release | 1 | 24.753 ±0.21 | 26.674 ±0.21 | 29.548 ±1.45 | 21.81±0.39 | 27.813 ±0.54 |
| 2 | 31.346 ±0.20 | 33.218 ±0.35 | 41.863 ±1.54 | 24.91±0.34 | 30.546 ±0.34 | |
| 3 | 39.293 ±0.28 | 46.423 ±0.28 | 57.134 ±0.46 | 32.74±0.33 | 39.293 ±0.33 | |
| 4 | 46.876 ±0.12 | 54.834 ±0.18 | 61.909 ±0.20 | 39.40±0.17 | 45.886 ±0.17 | |
| 5 | 55.592 ±0.26 | 61.853 ±0.19 | 66.800 ±0.38 | 45.81±0.45 | 56.492 ±0.45 | |
| 6 | 62.555 ±0.65 | 75.354 ±0.55 | 71.621 ±0.54 | 61.51±0.31 | 63.955 ±0.79 | |
| 7 | 93.121 ±0.29 | 83.11 ±0.54 | 78.383 ±1.05 | 77.21±1.20 | 67.765 ±1.49 | |
| 8 | 93.726 ±1.07 | 93.982 ±1.28 | 83.467 ±0.89 | 85.72±0.32 | 72.633 ±1.02 | |
| 9 | 94.035 ±0.67 | 94.184 ±1.40 | 95.255 ±0.44 | 93.82±0.29 | 82.102 ±0.99 | |
| 10 | 94.545 ±0.66 | 94.742 ±1.23 | 95.310 ±0.32 | 94.20±1.08 | 94.401 ±0.42 | |
| 11 | 94.931 ±1.17 | 94.949 ±0.74 | 95.422 ±0.40 | 94.72±1.21 | 94.719 ±0.18 | |
| 12 | 94.960 ±0.43 | 95.558 ±0.55 | 95.556 ±1.64 | 94.78±0.82 | 94.849 ±0.14 | |
| Production
yield (%) |
31.55 | 35.25 | 44.25 | 33.74 | 42.98 | |
| Encapsulation efficiency (%) | 60.24 | 71.44 | 80.11 | 72.14 | 79.55 | |
| Swelling
index (%) |
204±8 | 212±6 | 260±5 | 158±4 | 169±4 | |
| Formulations | |||||
| F6 | F7 | F8 | F9 | ||
| *Percent drug release | 1 | 25.33±0.31 | 26.003 ±0.14 | 24.512 ±1.16 | 24.619 ±0.45 |
| 2 | 36.00±0.32 | 37.253 ±0.80 | 29.013 ±0.41 | 27.721 ±0.52 | |
| 3 | 46.81±0.34 | 53.213 ±1.04 | 33.332 ±0.25 | 32.561 ±1.37 | |
| 4 | 67.36±0.42 | 65.403 ±0.29 | 38.429 ±0.17 | 38.557 ±0.41 | |
| 5 | 71.18±0.08 | 76.212 ±0.23 | 47.391 ±0.24 | 46.655 ±0.65 | |
| 6 | 76.85±0.51 | 84.624 ±0.17 | 53.882 ±0.92 | 53.780 ±0.79 | |
| 7 | 81.83±0.31 | 94.110 ±0.77 | 65.778 ±1.23 | 65.721 ±1.49 | |
| 8 | 85.11±1.64 | 94.252 ±0.27 | 69.706 ±0.35 | 69.594 ±1.02 | |
| 9 | 86.71±0.59 | 94.457 ±0.62 | 77.517 ±1.06 | 76.741 ±0.99 | |
| 10 | 95.59±0.59 | 95.106 ±0.44 | 83.532 ±0.39 | 83.195 ±0.41 | |
| 11 | 95.66±0.54 | 95.553 ±0.61 | 95.273 ±1.87 | 88.544 ±0.24 | |
| 12 | 95.80±0.19 | 95.778 ±0.44 | 95.410 ±1.51 | 96.146 ±0.45 | |
| Production yield (%) | 51.65 | 39.54 | 55.64 | 62.95 | |
| Encapsulation efficiency (%) | 88.12 | 73.25 | 80.54 | 92.57 | |
| Swelling
index (%) |
175±6 | 118±5 | 130±4 | 148±4 | |
Discussion: In vitro dissolution release study of the microspheres indicates that Formulation f1 is combination of 1:2:0 Flurbiprofen : Na-alginate : Na-CMC and gluteraldehyde 0.0 % shows 100% release upto 7.0 h. f2 is combination of 1:2:1 Flurbiprofen : Na-alginate : Na-CMC & gluteraldehyde 0.0 % shows 100% release upto 8.0 h. f3 is combination of 1:2:2 Flurbiprofen : Na-alginate : Na-CMC & cross linking agent 0.0 % shows 100% release upto 9.0 h.
Formulation f4 is combination of 1:2:0 Flurbiprofen : Na-alginate: Na-CMC and gluteraldehyde 20.0 % shows 100% release upto 9.0 h f5 is combination of 1:2:1 Flurbiprofen : Na-alginate : Na-CMC & gluteraldehyde 20.0 % shows 100% release upto 10 h f6 is combination of 1:2:2 Flurbiprofen : Na-alginate : Na-CMC & gluteraldehyde 20.0 % shows 100% release upto 10 h.
Formulation f7 is combination of 1:2:0 Flurbiprofen : Na-alginate: Na-CMC and glutaraldehyde 30.0 % shows 100% release up to 7h f8 is combination of 1:2:1 Flurbiprofen : Na-alginate: Na-CMC & glutaraldehyde 30.0 % shows 100% release up to 11h f9 is combination of 1:2:2 Flurbiprofen : Na-alginate: Na-CMC and glutaraldehyde 30.0 % shows 100% release up to 12h. From the above discussion, it was clear that as we increases the concentration of polymer & glutaraldehyde release of the drug was retarded.
From the above discussion formulation, f9 was the optimized formulation.
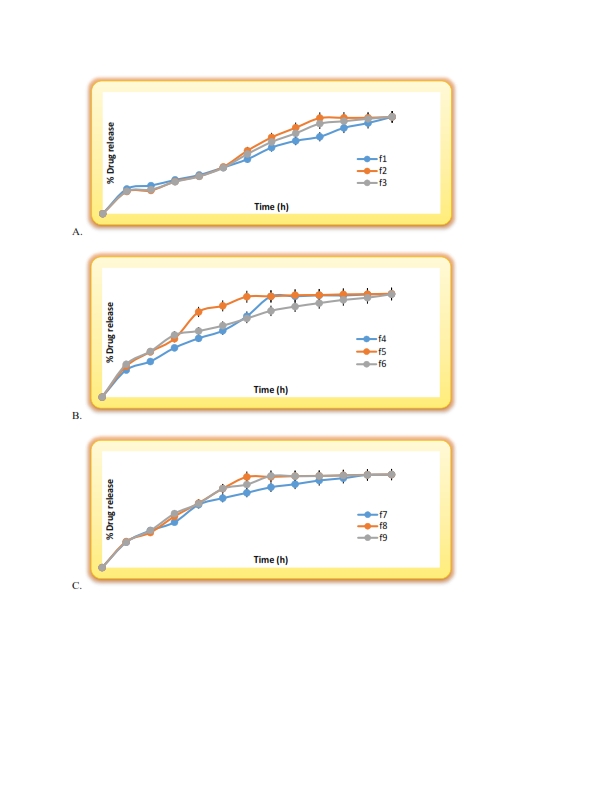
Figure.1 Dissolution profile of A. F.1-F.3, B. F.4-F.6, C. F.7-F.9 formulations for factorial batches.
The yield of production, Actual drug content, and entrapment (encapsulation) efficiency
The production yields of microspheres prepared through the spray dry technique are found in the range of 30-62%. Drug content (Actual) & drug encapsulation efficiency or drug entrapment efficiency of microspheres prepared by spray dry technique was found to be 60-92%.
In vitro mucoadhesive strength determination
Table.4 In vitro data for mucoadhesive strength estimation
| SR. NO | WEIGHT (mg) OF MICROSPHERES REMAINING ON GASTRIC MUCOSA | % MUCOADHESIVE STRENGTH | |||
| Optimized | 3h | 6h | 9h | 12h | |
| F9 (Spray dry) | 44 | 41 | 35 | 32 | 76.00 |
In vivo mucoadhesive strength determination
Table.5 In vivo data for mucoadhesive strength determination
| Sr.no | Weight (mg) of microspheres remaining on the rat stomach | % mucoadhesive strength | |||
| Time (h) | |||||
| Optimized | 0 | 4 | 8 | 12 | |
| Spray dry | 98.12 | 80.34 | 74.21 | 64.32 | 78.24 |
From both, in vitro & in vivo mucoadhesive strength determination tests it was cleared that Spray dry formulation comprising of 1:2:2 ratio of flurbiprofen: Sodium alginate: Sodium CMC it reduces the release of drug up to 12 hrs due to high mucoadhesive strength
Morphology of microspheres
Morphological study of microspheres done using SEM & microspheres was studied which shows the shape of microspheres almost spherical shown in fig no.2 and size shown in table no.6

Fig.2 Morphology of microspheres prepared by Spray dry
Shape & size of optimized formulations
Table.6 Shape & size of optimized formulations.
| FORMULATIONS | SIZE in µm | SHAPE |
| SIZE in µm(Spray dry) | 11.32-12.50 | Almost spherical |
Results of release parameters as T50%, T90%, and flurbiprofen release at 8h for spray dry method
Table.7 Results of release parameters
| Formulation | T90%
(h) ± SD (n-3)
|
T50%
(h) ± SD (n-3)
|
Flurbiprofen release at 8h
(%) ± SD (n-3) |
| F1 | 5.254± 1.01 | 5.354 ± 0.89 | 93.726 ±1.07 |
| F2 | 4.187 ± 1.45 | 4.498 ± 0.51 | 93.982 ±1.28 |
| F3 | 5.265 ± 0.57 | 4.884 ± 0.78 | 83.467 ±0.89 |
| F4 | 1.61 ± 1.21 | 1.65 ± 1.66 | 85.72±0.32 |
| F5 | 3.659 ± 0.92 | 4.305 ± 0.45 | 72.633 ±1.02 |
| F6 | 4.95 ± 0.78 | 7.006 ± 1.44 | 85.11±1.64 |
| F7 | 3.871 ± 1.32 | 5.546 ± 0.54 | 94.252 ±0.27 |
| F8 | 4.123 ± 0.78 | 6.45 ±1.22 | 69.706 ±0.35 |
| F9 | 4.316 ± 0.66 | 6.206 ± 1.02 | 69.594 ±1.02 |
Optimization of mucoadhesive microspheres formulations
A. Effect of formulation variables.
a.Effect of formulation variables on T50%
Model terms for response Y1 (T50%) were found to be significant with the F value of 4.88 (p<0.0048). All factors found significant in this study & model describing T50% can be written as;
Y1 = 2.87 + 0.51X1– 0.28 X2 + 0.26 X1 X2 + 0.48 X12 + 1.14 X22
As the amount of X1 and X2 increases the corresponding T50% also increases Fig 3 shows the response surface plot. It indicates at all the high levels of X1 and X2 the T50% value is high, As discussed above this behavior is due to an increase in the number of polymers (Na-alginate and Na- CMC) & cross-linking agent forms a high viscous gel matrix and thus decreases the drug release and hence T50% value increases, while Sodium CMC forms pores in the formed matrix and will increases the drug release thus decreases the T50% value. Fig 4 shows the graph of predicted versus actual data.
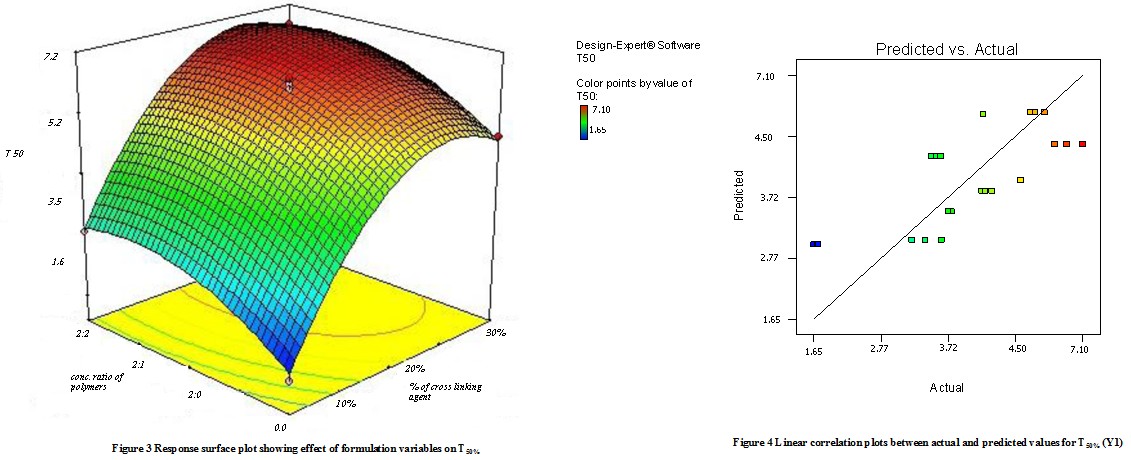
b.Effect of formulation variables on T90%
Model terms for response Y2 (T90%) were found to be significant with an F value of 10.11 (p<0.0001). All factors found significant in this study & model describing T50% can be written as;
Y2 = -5.79 + 0.68X1 – 14.83X2 + 0.99 X1X2 + 15.32 X12 + 16.12 X22
As the amount of X1 and X2 increases the corresponding T90% (time required to release 90% of the drug) also increases Fig 5 shows the response surface plot. It indicates at all the high levels of X1 and X2 the T50% value is high, As discussed above this behavior is due to an increase in the amount of polymers (Na-alginate and Na- CMC) & cross-linking agent forms a high viscous gel matrix and thus decreases the drug release and hence T50% value increases, while Sodium CMC forms pores in the formed matrix and will increases the drug release thus decreases the T90% value. Fig 6 shows the graph of predicted versus actual data.
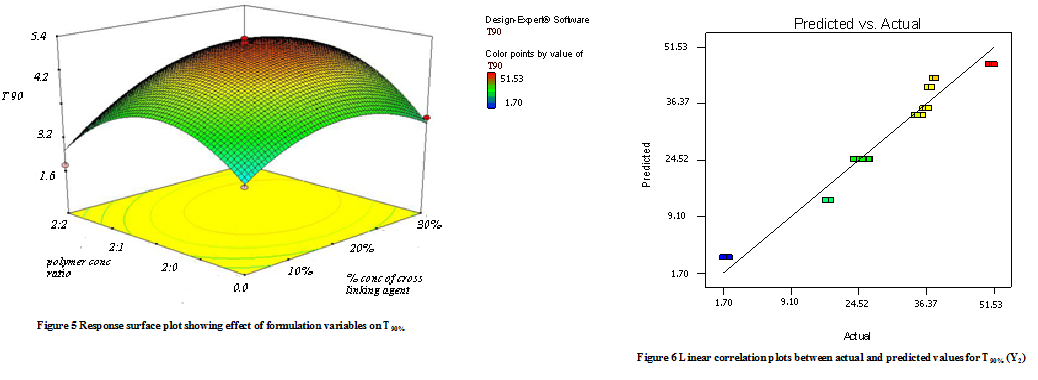
C. Effect of formulation variables on the drug release at 8 hr. (Y3)
The quadratic model was found to be significant with an F value of 28.22 (P<0.0001). In this case, X1, and X2 were found to be significant & model describes the percent flurbiprofen release at 8h can be written as;
Y3 = 81.76 – 0.29X1 + 11.45 X2
As the concentration of mucoadhesive polymer (Na-alginate and Na- CMC) enhances it causes a rise in the viscosity of the swollen matrix(gel), it contributes more hindrance to drug diffusion & thus reduces the release rate. The combined effect of X1 & X2 is shown in the response surface plot (Fig 7) In this plot it was observed that the increasing amount of Na- CMC causes the decreases in the drug release, because of the formation of gel(high viscosity)matrix. The factors X1 & X2 have a negative effect on the drug release. Fig 8 shows a graph of observed versus predicted values. The sodium alginate and Na- CMC have -ve effect on drug release, due to enhanced viscosity & gel strength. The swelling of sodium alginate may be because of the uncharged –COOH group which forms H- bonds with imbibing water & also holds water inside the gel matrix. An increasing amount of Sodium CMC forms a gel matrix network with sodium alginate.
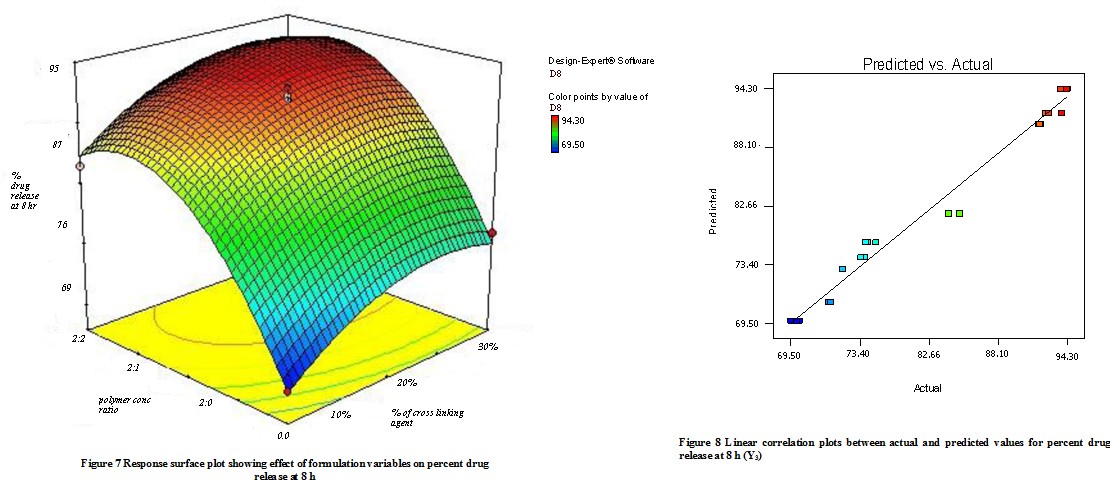
ANOVA., Pure error., Lack of fit
Results of the ANOVA shown in Table 9 model found significant for all responses (variables). Regression coefficient obtained by regression analysis. (Table 10) & effects are as follows; all factors were found to be significant for response Y1, similarly only X1, X2, and X1X2 were found for Y2, the X1, and X2 were found significant for Y3. The above results conveyed to us that the amount of sodium alginate, Sodium CMC plays important role in the formulation of mucoadhesive microspheres of flurbiprofen. Thus suitable range with these variables (yields) and optimized mucoadhesive microspheres have good strength (bioadhesive) & drug release. The predicted data of pure error & lack of fit are given in Table 9 Residuals are the difference in observed value & predicted value. Since computed F-values were respectively less than critical F values, denotes non-significance of lack of fit.
Table.9 Data of ANOVA study for dependent variables from 32 factorial design
| Source | d.f. | Sum square | Mean square | F value | Probability |
| T50% (h)
X1 X2 X1X2 |
1 1 1 |
5.08 1.48 0.87 |
5.08 1.48 0.87 |
7.56 2.20 1.30 |
0.0120 0.1526 0.2677 |
| T90% (h)
X1 X2 X1X2 |
1 1 1 |
8.28 3959.58 11.80 |
8.28 3959.58 11.80 |
0.060 28.66 0.085 |
<0.0001 <0.0001 0.0009 |
| NF release at 8 h (%)
X1 X2 |
1 1 |
1.60 1862.21 |
1.60 1862.21 |
0.047 54.83 |
0.8298 <0.0001 |
Table.10 Data of ANOVA study for results in analyzing lack of fit and pure
| Source | d.f. | Sum square | Mean square | F value | Probability |
| T50% (h)
Model Residual Total Lack of fit Pure error |
5 21 26 3 18 |
15.89 14.10 30.00 13.82 0.28 |
3.18 0.67 —– 4.61 0.016 |
4.88 —– —– 295.79 —– |
0.0048 —– —– <0.0001 |
| T90% (h)
Model Residual Total Lack of fit Pure error |
5 21 26 3 18 |
6948.06 2901.00 9849.06 2900.00 1.00 |
1389.61 138.14 —– 966.67 0.056 |
10.11 —– —– 17347.34 —– |
<0.0001 * —– —– <0.0001 |
| NF release at 8 h (%)
Model Residual Total Lack of fit Pure error |
2 24 26 6 18 |
1863.81 815.18 2678.99 804.28 10.90 |
931.91 33.97 —– 134.05 0.61 |
28.22 —– —– 221.14 —– |
<0.0001 * —– —– <0.0001 |
Optimization
As per the use of the optimization technique desirability, modes were used to generate an optimum solution for preparation. The process was optimized for variables (dependent) Y1-Y4. The optimized formula generated by targeting the Y1 was targeted at 6 h, Y2 was targeted at 10 h, and Y3 was kept at the range of 70-80% drug release. The optimized results obtained to give 7 results out of that one formula are shown in Table 11. Results of the optimized formula were compared with the predicted values, which showed match data between experimented & predicted values, which confirms the practicability & validity of the model.
Table.11 Composition of optimized formulation
| Ingredients | Quantities (mg) |
| Drug: Sodium alginate: Sodium CMC
% of cross-linking agent |
1:2:2
30 |
SUMMARY & CONCLUSION:
The results so far obtained during this investigation encouraged us to derive the following conclusions
- The yield of production of microspheres prepared by the spray drying method was found in the range of 30-62 % which is reliable
- The encapsulation efficiency of microspheres prepared by spray drying method was found in the range of 60-92% it is not 100% because during the preparation of microspheres some drugs were lost in external media.
- The in vitro release profile of Flurbiprofen from optimized formulations in spray drying technique where F9 shows retardation of release up to 12 hours shows good controlled release.
- The in vitro Flurbiprofen release data best fitted to the Korsmeyer-Peppas release model & also shows zero order & Higuchi model.
- The in vitro mucoadhesive strength of optimized formulations of spray drying technique F3 is 76.50% which shows good mucoadhesion.
- The in vivo mucoadhesive strength of optimized formulations of spray drying technique was for F9 78.24% which shows good mucoadhesion.
- The size of microspheres prepared by the spray drying method was found for F9 11.32-12.50µm
REFERENCES:
- Gelbert S. Banker, Christopher T. Rhodes.1996, Modern Pharmaceutics, New York Marcel Dekker, fourth edition,:. 501-516.
- James C.B., James S., Yie W.C., 2005, Encyclopedia of pharmaceutical technology, 2nd Edition, vol.-1 sengshang lin., New York,: 810.
- Brahamankar D M, Jaiswal S B.2005, Biopharmaceutics & Pharmacotherapeutics, Vallabh Prakashan,:. 335-339
- Jain N K.2008, Advances in controlled and novel drug delivery, CBS publication,:.1-10, 13-18, 19
- Mathiowitz E, Chickering, D. E, D.E., 1992, Definition, Mechanisms and Theories Of Bioadhesion, in; E. Mathiowitz, D.E. Chickering, C.M. Lehr(eds), Bioadhesive drug delivery system: fundamentals, novel approaches, and development, Marcel Dekker, New York,: 1-10,16-19.
- Das M. K, Senapati. P. C. 2004, Furosemide Loaded AlginateMicrosphere prepared by Ionic Cross linking Technique Morphology & Release Characteristics,: 122-125 63. Martin A.2004, In Micromeritics, Physical Pharmacy. New Delhi, India: Waverley Pvt Ltd; 1999: 423-436
- Singh B, Chakkal S K, and Ahuja N.2006, Formulation and Optimization of Controlled Release Mucoadhesive Tablets of Atenolol Using Response Surface Methodology. AAPS PharmSciTech. ; 7(1):. 34-38
- Sastry SV, Reddy IK, Khan MA.1997, Atenolol gastrointestinal therapeutic system: optimization of formulation variables using response surface methodology. J Control Release.;45:121-130.
- Trivedi P, Verma AML,2007, Garud N.Preparation and evaluation of aceclofenac microspheres.Asian journal of pharmaceutics 2; 110-115
- Thakkar PH, Murthy RR.2008, Effect of crosslinking agent on characteristics of celecoxib loaded chitosan microspheres. Asian journal of pharmaceutics 2; 246-251
11. Radhika PR, Borkhataria CH.2008, Preparation and evaluation of delayed-release aceclofenac microspheres. Asian journal of pharmaceutics 3; 252-254
- Hu-Lin Jiang, Rohidas Arote, Ji-Shan Quan, Mi-Kyong Yoo, You-Kyoung Kim, In-Yong Kim, Zhong-Shan Hong, Hong-Gu Lee, Xun Jin, Yun-Jaie Choi, Chong-Su Cho.2001, Alginate-Coated Thiolated Chitosan Microspheres for an Oral Drug Delivery System In Vitro, Key Engineering Materials Vols. 342-343),: 433-436
- Delie F, Blanco-Prieto M (2005), Polymeric particulates to improve oral bioavailability of peptide drugs. Molecules. 10: 65-80.
- Indian pharmacopeia. 2007. Government of India, Ministry of health and family welfare, Vol-3, published by the controller of publications, The Indian pharmacopeia commission Ghaziabad, 1442-1445
- Kohali DPS, Shah DH.1999, Drug formulation manual, eastern publication,:. 475
- Gowda D.V, Shivkumar H.G.2004, Preparation & Evaluation of Waxes/Fat Microsphere Loaded with Lithium carbonate for Controlled release, AAPS PharmSciTech; 5 (4) Article 67,: 67-70
- Yilmaz Capan, Ge Jiang, Stefano Giovagnoli, Kyu-Heum Na, and Patrick P. DeLuca.2004, Preparation and Characterization of Poly(D,L-lactide-co-glycolide) Microspheres for Controlled Release of Human Growth Hormone, AAPS PharmSciTech ; 4 (2) Article 28,: 56-67.
- Matthew P. Deacon., Simon Mcgurk., Clive J. Roberts, Phillip M. Wiliams., Saul J. B. Tendler., Martyn C. Davies., S. S. (Bob) Davis. and Stephen E. Harding.2007, Atomic force microscopy of gastric mucin and chitosan mucoadhesive systems, Biochem. J.:348,557
- Hu-Lin Jiang, Rohidas Arote, Ji-Shan Quan, Mi-Kyong Yoo, You-Kyoung Kim, In-Yong Kim, Zhong-Shan Hong, Hong-Gu Lee, Xun Jin, Yun-Jaie Choi, Chong-Su Cho.2001, Alginate-Coated Thiolated Chitosan Microspheres for an Oral Drug Delivery System In Vitro, Key Engineering Materials Vols. 342-343),: 433-436
- Basavraj BV.2008, Hallow microspheres of diclofenac sodium A gastroretentive controlled drug delivery system, Pak. J. Pharm. Sci., Vol.21, No.4, 451-454
- 21. Anande N M., Jain S K., Jain N K.,.2008, Con-A conjugated mucoadhesive microspheres for the colonic delivery of diloxanide furoate, International Journal of Pharmaceutics 359,: 182–189
- Dhaliwal S, Jain S, Singh H P, and. Tiwary A. K.2008, Mucoadhesive Microspheres for Gastroretentive Delivery of Acyclovir In Vitro and In Vivo Evaluation, The AAPS Journal, .34-42.
- Ghiasi1 Z., Sajadi S. A,. Tafaghodi M. 2004, Preparation and In Vitro Characterization of Alginate Microspheres Encapsulated with Autoclaved Leishmania major (ALM) and CpG –ODN. Iranian Journal of Basic Medical Sciences Vol. 10, No. 2, Summer 200: 90-98
- Kostanski J W, Dani B A., Reynolds G A, Bowers C. Y, and DeLuca P P.2001, Evaluation of Orntide Microspheres in a Rat Animal Model and Correlation to In Vitro Release Profiles, AAPS PharmSciTech, ; 1 (4) article: 87-94
- Capan Y, Jiang G, Giovagnoli S, Kyu-Heum N, and DeLuca P P.2003, Preparation and Characterization of Poly(D, L-lactide-co-glycolide) Microspheres for Controlled Release of Human Growth Hormone. AAPS PharmSciTech ; 4 (2)23-30.
